Yinyi®Drug-loaded coronary stent system without polymer micro-blind holes
Product Description
Yinyi® polymer-free microblind drug-loaded (paclitaxel) coronary stent (hereinafter referred to as "Yinyi® stent")
is a new generation of drug stent independently developed and produced by Yinyi Biotechnology.
The innovative drug delivery concept provides a safe, effective and economical treatment plan for patients with coronary artery stenosis.
Features
Traditional drug stents use high-molecular polymer carriers to carry drugs. With their widespread clinical use, many problems have been exposed, mainly as follows:
●Late stent thrombosis caused by delayed endothelial healing
●Late stage catch-up of restenosis caused by polymer chronic irritation and inflammation
●Acquired late adhesion
●Therefore, patients need to take long-term anticoagulant drugs to cause more bleeding risk
The current effective means to solve these problems is to develop a new generation of polymer-free drug stents.
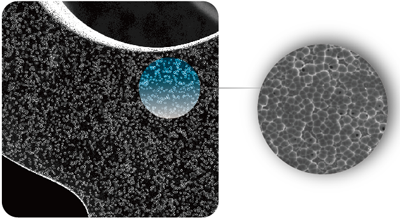
micro-scale blind holes to load paclitaxel on the metal surface, combining with the highly fat-soluble characteristics of paclitaxel.
Because there is no stimulating factor of high molecular polymer carrier, vascular endothelial cells are easier to cover the stent,
Innovative microblind hole drug loading method
Patented technology realizes micro-blind hole polymer-free drug loading
●The depth of the blind hole is less than 500nm, which is only 1/200 of the thickness of the stent
●The pore diameter is 1~2μm, which is more than a thousand times larger than the paclitaxel molecule (<1nm)
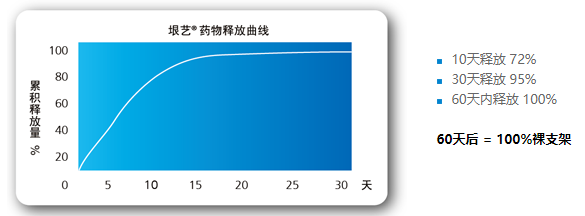
Effective drug release
The microblind hole drug loading combined with the fat-soluble characteristics of paclitaxel,
the drug release and the effect of inhibiting cell proliferation achieve the best combination.
Green natural medicine
Paclitaxel, which is naturally extracted from the bark and branches of Taxus chinensis, is an internationally recognized first-line anti-tumor drug.
A large number of clinical applications have proved that the paclitaxel stent has more obvious therapeutic advantages than the rapamycin stent in the following aspects:
●Suitable for vascular disease caused by coronary heart disease combined with diabetes
●Suitable for the treatment of in-stent restenosis caused by rapamycin stent
●Suitable for patients allergic to rapamycin
Higher cost performance
Yinyi® stent brings higher cost performance to patients, mainly reflected in:
●It is an upgraded alternative product of polymer carrier drug stent
●It has better curative effect than rapamycin stent in the treatment of coronary heart disease with diabetes
●Patients do not need to take anticoagulant drugs for a long time, reducing the patient's postoperative drug expenditure
Clinical validation
The Yinyi® stent has been proven to be safe and effective after a number of large-scale clinical studies and extensive applications.
●1 pre-marketing study (nearly 170 cases)
●2 large clinical studies (1045 cases and 1626 cases respectively)
●Implant use in 150,000 patients in 6 years
●Widely used in more than 400 hospitals
●It is currently the most used non-polymer coated drug stent in the world
Product Honor
●In 2007, the product obtained the SFDA certificate, which is the first non-polymer-coated drug stent in China
●In 2008, the product obtained the national authorized invention patent (ZL200610109422.8)
●In 2008, the product was appraised as the four highlights of my country’s medical device research and development by the State Food and Drug Administration’s “China Medical News”, and is considered to be an internationally leading drug stent
●In 2008, the product was introduced by the "Guangming Daily", "Technology Daily" and other large domestic media
●In 2009, the product obtained the certificate of independent innovation product
●In 2011, the product won the first prize of Dalian Technical Invention
●In 2012, the microblind hole drug-loading patent won the Liaoning Provincial Patent Excellence Award
Product Specification
|
支架长度 (mm) |
支架直径 (mm) | ||||
| 2.50 | 2.75 | 3.00 | 3.50 | 4.00 | |
| 8 | DSS2508 | DSS2708 | DSS3008 | DSS3508 | DSS4008 |
| 10 | DSS2510 | DSS2710 | DSS3010 | DSS3510 | DSS4010 |
| 12 | DSS2512 | DSS2712 | DSS3012 | DSS3512 | DSS4012 |
| 15 | DSS2515 | DSS2715 | DSS3015 | DSS3515 | DSS4015 |
| 18 | DSS2518 | DSS2718 | DSS3018 | DSS3518 | DSS4018 |
| 21 | DSS2521 | DSS2721 | DSS3021 | DSS3521 | DSS4021 |
| 23 | DSS2523 | DSS2723 | DSS3023 | DSS3523 | DSS4023 |
| 26 | DSS2526 | DSS2726 | DSS3026 | DSS3526 | DSS4026 |
| 28 | DSS2528 | DSS2728 | DSS3028 | DSS3528 | DSS4028 |
Registration Message